FILLERVIT OX
FILLING AND MODELLING SOLUTION OF THE DERMAL MATRIX BASED ON HYALURONIC ACID AND ANTIOXIDANTS
Areas of application: Face, neck, décolleté and hands.
Fillervit OX
PRODUCTClass III medical device with CE mark issued by the National Institute of Health and containing: fragments of hyaluronic acid (20-38 monomers), amino acids, reduced glutathione, sodium ascorbyl phosphate, phosphate-based buffer system.
ACTION
The method is called Skin Improvement and has the function of optimizing skin physiology through fibroblastic activation for the neo-formation of matrix components and for the normalization of the colloidal state of the matrix, as well as to prevent damage from chrono-ageing caused by the free radicals.
The Medical Device used for this purpose contains:
• fragments of hyaluronic acid (20-38 monomers) capable of activating CD44 of fibroblast
• amino acid precursors for collagen, elastin and glycosaminoglycans
• antioxidants for the prevention of free radical damage
The product should be administered intra dermally according to the primary mesotherapy injecting technique.
PRINCIPLE OF METHOD
CD44 of the fibroblasts are activated and the cells in the surrounding area are provided withthenecessaryprinciplesforthebuild-upofcollagen,elastinandglycosaminoglycans. Biological optimization of skin condition will be obtained. In addition, acid buffering of the matrix maintains the sol status of the colloidal solution and facilitates the metabolic exchanges. Eventually, the antioxidants block the free radicals and allow the prevention of skin damages due to aging.
FILLERVIT C
FILLING AND MODELLING SOLUTION OF THE DERMAL MATRIX BASED ON HYALURONIC ACID WITH ADDED CHOLINE
Fillervit C
PRODUCT
Class III medical device with CE mark issued by the National Institute of Health and containing: fragments of hyaluronic acid (20-38 monomers), amino acids, phosphate-based buffer system with added choline.
ACTION
The technique is defined Skin Improvement with the aim to optimize skin physiology through fibroblast activation for the neo-formation of matrix components and for the normalization of the colloidal state of the matrix as well as to prevent skin damage by photo-ageing.
Class III medical device with CE mark issued by the National Institute of Health and containing:
• fragments of hyaluronic acid (20-38 monomers) capable of activating CD44 of fibroblast
• amino acid precursors for collagen, elastin and glycosaminoglycans
• phosphate-based buffer system
The product should be administered intradermally according to the primary mesotherapy injecting technique.
PRINCIPLE OF METHOD
CD44 of the fibroblasts are activated and the cells in the surrounding area are provided with the necessary principles for the build-up of collagen, elastin and glycosaminoglycans. Biological optimization of skin condition will be obtained. In addition, acid buffering of the matrix maintains the sol status of colloidal solution and facilitates the metabolic exchanges. Finally, Choline, as a precursor for acetylcholine, optimizes the cholinergic cutaneous system and the corneocytarious differentiation with the prevention of damages due to UV rays.
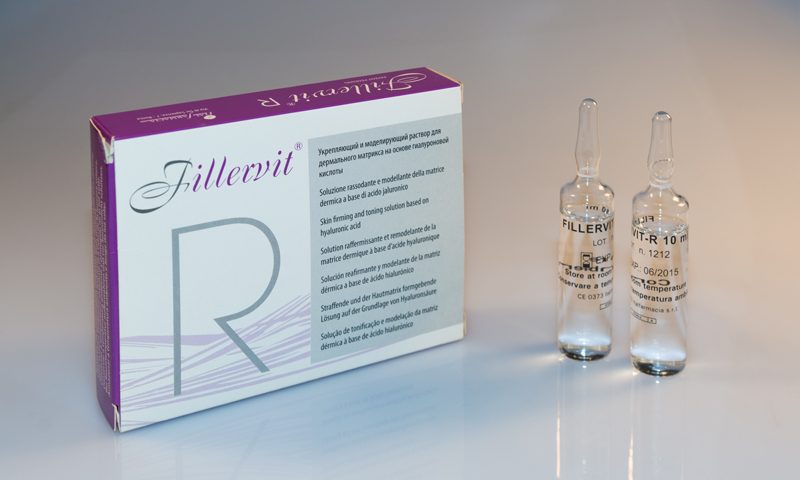
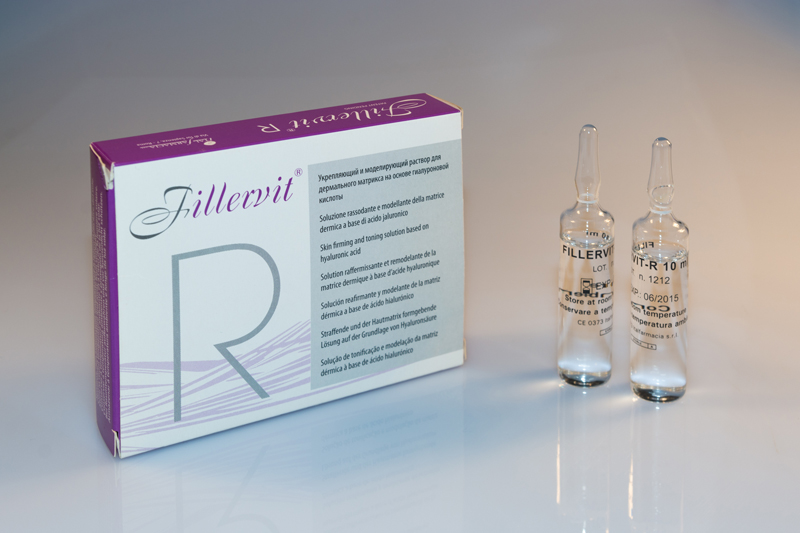
FILLERVIT R
FIRMING AND MODELLING SOLUTION OF THE DERMAL MATRIX
Fillervit R
PRODUCT
Class III medical device with CE mark issued by the National Institute of Health and containing: hyaluronic acid and hyperosmolar solution of acidic pH.
ACTION
Bio-restoration is performed through the activation of fibroblast subpopulation of fibrotic fibroblast in order to increase skin firmness with deposition of cicatrizing collagen (Type 1).
The Medical device used for this purpose contains:
• fragments of hyaluronic acid (20-30 monomers) capable of activating CD44 of fibroblasts
• acid and hypertonic solution able to induce an irritating reaction and stimulate fibrotic fibroblasts
• amino acid precursors for collagen
Treatment should be performed on hypotonic tissues (face, neck, décolleté, buttock, thigh, arm, epigastric region).
FILLERVIT B
FILLING AND MODELLING SOLUTION OF THE DERMAL MATRIX BASED ON HYALURONIC ACID
Fillervit B
PRODUCT
Class III medical device with CE mark issued by the National Institute of Health and containing: fragments of hyaluronic acid (20-38 monomers), amino acids, buffer system based on bicarbonate.
ACTION
At present, the most commonly performed medical procedure for improving skin physiology is the bio-stimulation that provides fibroblast activation for the build-up of new matrix components and the normalization of the colloidal state of the matrix.
The Medical Device used for this purpose contains:
• fragments of hyaluronic acid (20-38 monomers) capable of activating CD44 of fibroblast
• amino acid precursors for collagen, elastin and glycosaminoglycans
• buffer system based on
The product should be administered intradermally according to the primary mesotherapy injecting technique.